Prenumeration
Du har en aktiv prenumeration.
Prenumerera på pressmeddelanden från Novakand Pharma via email.
Du prenumererar på följande språk.
Välj vilka språk du vill prenumerera på.
Modular Finance AB kommer att hantera vissa av dina personuppgifter om du väljer att prenumerera. Mer information om vår personuppgiftshantering finns här.
Intresserad av bolagets nyckeltal?
Analysera bolaget i Börsdata!
Vem äger bolaget?
All ägardata du vill ha finns i Holdings!

2023-03-21 08:30:00
The Japan patent office has issued a patent that protects the manufacturing processes for KAND567 and KAND145
Kancera AB (publ) today reports that the Japan patent office has issued a patent that protects the manufacturing processes for Kancera’s Fractalkine blocking drug candidates KAND567 and KAND145

2023-03-01 08:30:00
Kancera reports that patient enrollment to the FRACTAL-study is completed and that top line results will be presented in Q3 2023
Kancera AB (publ) today reports that the patient enrollment to the ongoing FRACTAL-study, a phase IIa study of KAND567 in myocardial infarction patients, has been completed and that a total of 71 patients have been recruited. Kancera aims to report top line data during Q3 2023

2023-02-16 14:50:00
Kancera reports that the Swedish Medical Products Agency has approved the application to conduct the KANDOVA-study and that Dr. Hanjing Xie has been appointed to Chief Medical Officer
Kancera AB (publ) today reports that the Swedish Medical Products Agency has approved the application to conduct the KANDOVA-study, a clinical study of KAND567 in ovarian cancer patients. The company further reports organizational changes, including the appointment of a new Chief Medical Officer

2023-01-30 08:32:00
Kancera provides operational update for its Fractalkine-blocking drug candidates KAND567 and KAND145
Kancera AB (publ) today reports that the clinical studies of its Fractalkine-blocking drug candidates KAND567 and KAND145 are progressing according to plans and that the US patent office has issued a patent that protects the manufacturing processes

2023-01-16 08:32:00
Kancera reports that the FRACTAL-study is on track to report top line results in Q3 2023
Kancera AB (publ) reports that 63 subjects have been recruited to the ongoing FRACTAL-study, a phase IIa study of KAND567 in myocardial infarction patients, which exceeds the initial objective of 60 study subjects in total. Kancera has previously announced the decision to amend the study protocol to allow for recruitment of up to 70 study subjects in total, in order to strengthen the study in term...

2022-05-23 22:10:00
Kancera provides operational update in connection with the interim report for the first quarter of 2022
This is a translation of a press release published in Swedish by Kancera AB (publ) on May 20, 2022

2022-05-20 08:35:00
Interim report first quarter 2022, 1 January – 31 March 2022 Kancera AB (publ.), org.nr. 556806-8851
First quarter in brief January – March Financial summary for the first quarter and the period January - March 2022 · Net sales amounted to SEK 0 million (SEK 0.0 million). · R&D costs amounted to SEK 10,6 million (8,2 million). · Operating profit for the first quarter amounted to SEK -11,9 million (-9,0 million). · Profit after financial items for the first quarter amounted to SEK -12,0 mill...

2021-12-07 08:40:00
Doktorsavhandling beskriver hur ROR-hämmare kan användas för behandling av cancer.
Under en doktorsavhandling med titeln ”ROR1 – a druggable target: preclinical studies of ROR1 and combinatorial partners in malignancies” presenterades prekliniska resultat som visar hur Kanceras ROR-hämmare kan komma att kombineras med standardläkemedel för att förbättra behandlingen av både blodcancer och solid cancer. Under disputationen försvarade Doktor Amineh Ghaderi på Karolin...
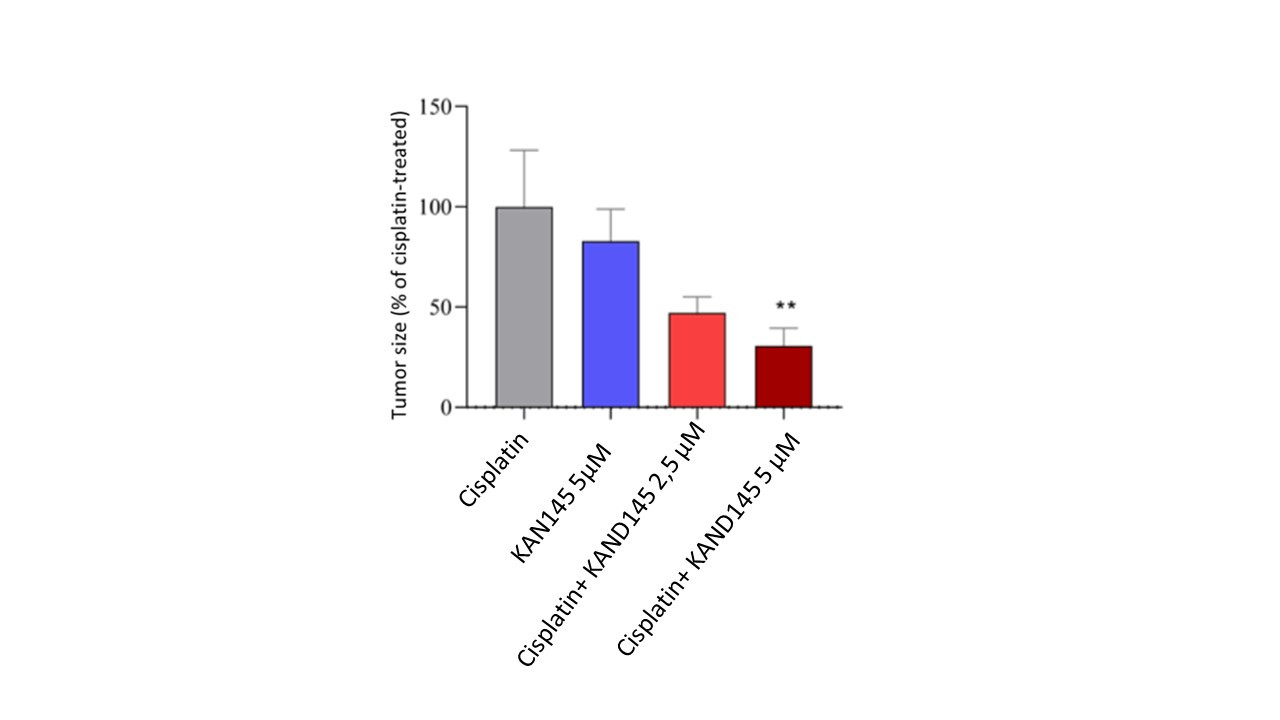
2021-11-22 18:33:00
Kancera’s drug candidate KAND145 effectively reduces tumor size in a preclinical model of ovarian cancer
This is a translation of press release in Swedish (2021-11-22). Kancera AB (Nasdaq First North Premier Growth Market: KAN) today presents preclinical results that show that KAND145 effectively reduces tumor size in a disease model of ovarian cancer. These results also provide guidance on which dose of KAND145 that may become effective against the tumor in future patient studies. Thus, Kancera has ...

2021-11-19 13:03:00
Kancera reports top-line data from the phase IIa study with KAND567 in COVID-19 patients
This is a translation of a press release in Swedish published 2021-11-18. Kancera AB (Nasdaq First North Premier Growth Market: KAN) presents results from the exploratory phase IIa study with KAND567 in patients with COVID-19. The randomized double-blinded study achieved the primary objective, which was to confirm that KAND567 has a favourable safety and tolerability profile also in severely ill p...

2021-06-16 22:30:00
Kancera has completed patient recruitment for the COVID-19 study with KAND567
Kancera AB (Nasdaq First North Premier Growth Market: KAN) announces that the recruitment of patients for the exploratory phase IIa study with KAND567 has ended after just over 80 percent of the originally planned number of COVID-19 patients have received their dose. The decision is made in light of the fact that the number of cases in need of hospital care has decreased significantly and the curr...

2021-05-24 13:35:00
Kancera announces that an application for a clinical phase IIa study with KAND567 after heart attack has been submitted to the UK Medicines Agency (MHRA)
Kancera AB (Nasdaq First North Premier Growth Market: KAN) announces today that the application for permission to start a phase IIa study evaluating the safety and cardioprotective effect of KAND567 in heart attack patients has been submitted to the UK Medicines and Healthcare Products Regulatory Agency, MHRA. The study will be conducted at Freeman Hospital under the direction of Professor Ioakim ...

2021-05-21 16:01:00
Interim report for first quarter 2021, 1 January – 31 March 2021
First quarter in brief Net sales for the period (January to March) amounted to SEK 0 million (SEK 0 million). R&D costs for the period amounted to SEK 8,2 million (SEK 9,7 million). Operating profit for the period amounted to SEK -9,0 million (SEK -11,1 million). Profit after financial items for the period amounted to SEK -9,1 million (SEK -11,7 million). Earnings per share for the period amounted...

2021-01-15 12:40:00
Kancera provides update on successful phase I study and planned phase II study with KAND567 treatment following heart attack
This is a translation of a press release in Swedish published December 29, 2020

2021-01-15 12:35:00
Kancera appoints a Scientific Advisory Board with leading scientists in cardiology and immunology
This is a translation of a press release in Swedish published December 21, 2020

2020-12-02 13:00:00
Agreement on development of HDAC6 inhibitors discontinued.
Kancera AB announces today that the pharmaceutical company Grünenthal GmbH has chosen to terminate the parties' research and option agreement. Since the end of 2018, Grünenthal has been responsible for the development of Kancera's series of HDAC6 inhibitors and made progress in the chemical development of the project. Kancera now takes over the rights to all results generated under the agreement...
