Beskrivning
| Land | Storbritannien |
|---|---|
| Lista | FTSE 100 |
| Sektor | Hälsovård |
| Industri | Läkemedel & Handel |
Intresserad av bolagets nyckeltal?
Analysera bolaget i Börsdata!
Vem äger bolaget?
All ägardata du vill ha finns i Holdings!
Trial met all secondary endpoints including change from baseline in quantitative myasthenia gravis total score at week four and week 26. Data reinforce potential of novel dual-binding nanobody gefurulimab to advance care as a self-administered subcutaneous treatment option for patients with AChR-Ab+ gMG.
Positive results from the global PREVAIL Phase III trial showed that gefurulimab met its primary endpoint, demonstrating a statistically significant and clinically meaningful improvement from baseline in Myasthenia Gravis Activities of Daily Living (MG-ADL) total score compared to placebo in adults with anti-acetylcholine receptor (AChR) antibody-positive (Ab+) generalised myasthenia gravis (gMG) at week 26. PREVAIL also met all secondary endpoints, including change from baseline in Quantitative Myasthenia Gravis (QMG) total score at week four and week 26.1
These data were presented at the Myasthenia Gravis Foundation of America (MGFA) Scientific Session during the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) Annual Meeting in San Francisco, California.1
Gefurulimab demonstrated improvement from baseline in MG-ADL total score at week 26 compared to placebo (treatment difference: -1.6 [95% CI: -2.4, -0.8], p<0.0001). A clinically meaningful improvement was observed as early as week one, and was sustained through week 26.1
Additionally, a clinically meaningful improvement in key secondary endpoint, QMG total score, was seen as early as week four (treatment difference: -1.8 [ 95% CI: -2.5, -1.1], p<0.0001) and was sustained through week 26 (treatment difference: -2.1 [95% CI: -3.1, -1.1], p<0.0001).1
Kelly Gwathmey, MD, Associate Professor of Neurology, Chief of Neuromuscular Division, Virginia Commonwealth University, Richmond, VA, Vice Chair of the MGFA Medical & Scientific Advisory Council and principal investigator in the trial, said: "People living with gMG face fluctuating and often debilitating symptoms, including loss of muscle function and severe weakness. Results from the PREVAIL Phase III trial demonstrating early and lasting benefits in MG-ADL and QMG scores support the potential for gefurulimab to offer an efficacious and convenient self-administered treatment option that may help address the unpredictability of this disease."
Gianluca Pirozzi, Senior Vice President, Head of Development, Regulatory and Safety, Alexion, AstraZeneca Rare Disease, said: "Findings from PREVAIL offer valuable insight into how early and sustained complement inhibition with gefurulimab may translate into meaningful, functional improvement for people living with gMG. Improvements reflected in both patient- and physician-reported outcome measures further underscore the clinical relevance of these results. As one of the largest global Phase III trials in patients with AChR-Ab+ gMG, PREVAIL data reflect our commitment to advancing rigorous, patient-centred science that can transform care for people living with this debilitating and unpredictable disease worldwide."
Summary of primary endpoint
LS mean change (95% CI) from RCT baseline in MG-ADL total score
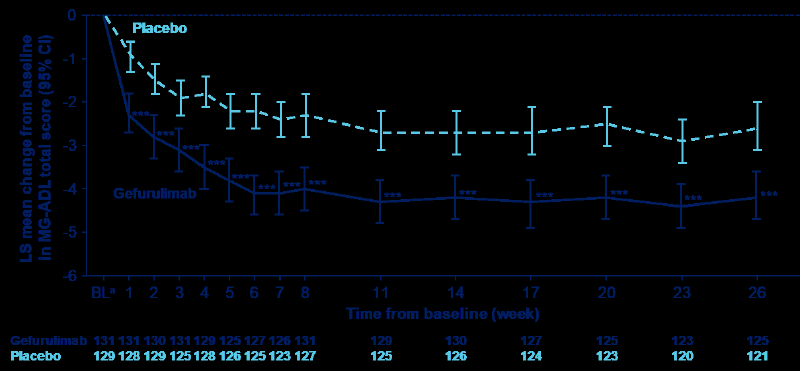
***P<0.001; 2-sided nominal P values are reported for weeks 1-23 for the comparison of change from baseline between treatment groups.
aBaseline is defined as the last available assessment value prior to the first dose of study intervention.
BL, baseline; LS least squares; MG-ADL, Myasthenia Gravis Activities of Daily Living; RCT, randomized controlled treatment;
SEM, standard error of the mean.
Results from the PREVAIL Phase III trial showed treatment with gefurulimab was well-tolerated, and the safety profile was consistent with previous trials of C5 inhibitors eculizumab and ravulizumab in gMG. The percentage of participants with treatment emergent adverse events (TEAEs) was similar between those who received gefurulimab and placebo, and most TEAEs observed were mild to moderate in severity (grade 1 or 2). The most common TEAEs were injection site reactions (9.9%), headache (9.9%), and back pain (7.6%) in those treated with gefurulimab and headache (12.4%), diarrhoea (8.5%), and upper respiratory tract infection (7.8%) in those who received placebo.1
Notes
gMG
gMG is a rare autoimmune disorder characterised by loss of muscle function and severe muscle weakness.2
Eighty-five percent of people with gMG are AChR antibody-positive meaning they produce specific antibodies (anti-AChR) that bind to signal receptors at the neuromuscular junction (NMJ), the connection point between nerve cells and the muscles they control.3 This binding activates the complement system, causing the immune system to attack the NMJ, leading to inflammation and a breakdown in communication between the brain and the muscles.4
gMG can occur at any age, but it most commonly begins for women before the age of 40 and for men after the age of 60.5 Initial symptoms may include slurred speech, double vision, droopy eyelids and lack of balance; these can often lead to more severe symptoms as the disease progresses such as, impaired swallowing, choking, extreme fatigue and respiratory failure.6,7
PREVAIL (ALXN1720-MG-301)
PREVAIL (ALXN1720-MG-301) is a global, Phase III, randomised, double-blind, placebo-controlled, parallel, multicentre study evaluating the safety and efficacy of gefurulimab in adults with generalised myasthenia gravis (gMG). The trial enrolled 260 patients from 20 countries across North America, Europe, Asia and the Pacific region. Participants were required to have a confirmed myasthenia gravis diagnosis at least three months prior to the screening visit with a positive serological test for autoantibodies against AChR and Myasthenia Gravis Foundation of America Clinical Classification Class II to IV at screening.8
Patients were randomised 1:1 to receive gefurulimab or placebo for a total of 26 weeks in the randomised controlled treatment period. Patients received a single weight-based loading dose on Day 1, followed by regular weight-based maintenance dosing beginning on Day 8 and once every week thereafter. The primary endpoint of the change from baseline in the Myasthenia Gravis Activities of Daily Living (MG-ADL) total score, a patient-reported scale that assesses patients' abilities to perform daily activities, was assessed at week 26 along with multiple secondary endpoints evaluating improvement in disease-related measures.8
Patients who completed the randomised controlled treatment period were eligible to continue into an open-label extension period evaluating the safety and efficacy of gefurulimab, which is ongoing.8
Gefurulimab
Gefurulimab, an investigational complement C5 inhibitor, is a novel dual-binding nanobody optimised for subcutaneous self-administration in development as a treatment for AChR-Ab+ gMG. The investigational medication works by binding to the C5 protein in the terminal complement cascade, a part of the body's immune system. When activated in an uncontrolled manner, the complement cascade over-responds, leading the body to attack its own healthy cells. Concurrent binding of gefurulimab to serum albumin provides an extended half-life, enabling once-weekly dosing. Gefurulimab has been granted Orphan Drug Designation in the US for the treatment of myasthenia gravis.
Alexion
Alexion, AstraZeneca Rare Disease, is focused on serving patients and families affected by rare diseases and devastating conditions through the discovery, development and delivery of life-changing medicines. A pioneering leader in rare disease for more than three decades, Alexion was the first to translate the complex biology of the complement system into transformative medicines, and today it continues to build a diversified pipeline across disease areas with significant unmet need, using an array of innovative modalities. As part of AstraZeneca, Alexion is continually expanding its global geographic footprint to serve more rare disease patients around the world. It is headquartered in Boston, US.
AstraZeneca
AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialisation of prescription medicines in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca's innovative medicines are sold in more than 125 countries and used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Social Media @AstraZeneca.
Contacts
For details on how to contact the Investor Relations Team, please click here. For Media contacts, click here.
References
- Gwathmey KG. Efficacy and safety of subcutaneous self-administered gefurulimab in generalized myasthenia gravis: topline results from a phase 3, randomized, double-blind, placebo-controlled study (PREVAIL). Presented at the Myasthenia Gravis Foundation of America (MGFA) Scientific Session at the 2025 American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) Annual Meeting; 2025 October 29; San Francisco, CA.
- Jung-Plath W, et al. Assessment of myasthenia gravis patients' quality of life. The Journal of Neurosurgical Nursing. 2023;12(2):74-83.
- Lazaridis K, et al. Myasthenia gravis: autoantibody specificities and their role in MG management. Front Neurol. 2020;11:596981.
- Huang YF, et al. Visualization and characterization of complement activation in acetylcholine receptor antibody seropositive myasthenia gravis. Muscle Nerve. 2024.
- Cavanagh N, et al. Exploring the impairments and allied health professional utilization in people with myasthenia gravis: a cross-sectional study. J Clin Neurosci. 2023;114:9-16.
- Catalin J, et al. Clinical presentation of myasthenia gravis. Thymus. 2019.
- Farid ZR, et al. Factors affecting generalization of ocular myasthenia gravis. Sriwijaya Journal of Ophthalmology. 2020;3(2):48-54.
- ClinicalTrials.gov. Safety and efficacy of ALXN1720 in adults with generalized myasthenia gravis. NCT Identifier: NCT05556096. Available here. Accessed October 2025.
